

Lokesh Ravilla, Parimala Hanumesh, Thammanna Gowda SS, Manjunath V Shinnur, Ajay Prabu B, Jayapraksh G, Sourabh SH and Shobith Rangappa
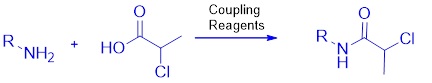
A streamlined approach for generating a precursor for prilocaine is presented. Revisiting the conversion of 2-chloropropanoic acid to 2-chloropanoyl chloride using thionyl chloride, the synthesis of the prilocaine derivative precursor has been explored using various coupling reagents and reaction conditions to improve yield. Optimal results were attained by utilizing 1.5 equivalents of HATU as the coupling reagent, in conjunction with 2 equivalents of 2-chloropropanoic acid, at a temperature of 35 °C. The synthesis of prilocaine amide required 14 hours under these conditions.
Graphical Abstract
Pages: 38-42 | 1869 Views 889 Downloads
